Product Information
Intended Use and Indications for the NephroCheck® Test System
The NephroCheck® Test System is intended to be used in conjunction with clinical evaluation in patients who currently have or have had within the past 24 hours acute cardiovascular and or respiratory compromise and are ICU patients as an aid in the risk assessment for moderate or severe acute kidney injury (AKI) within 12 hours of patient assessment. The NephroCheck® Test System is intended to be used in patients 21 years of age or older.
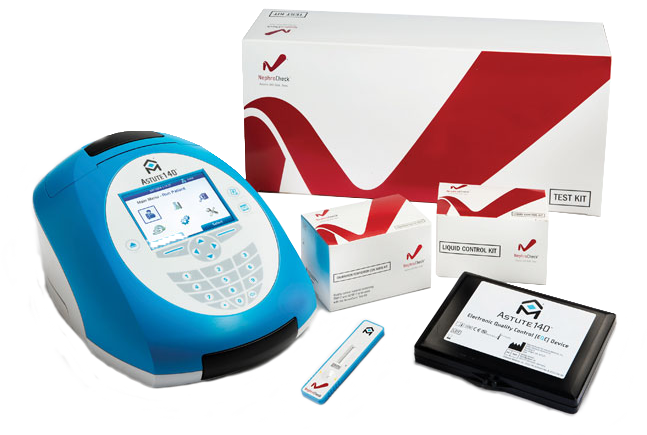
The NephroCheck® Test System includes the following components:
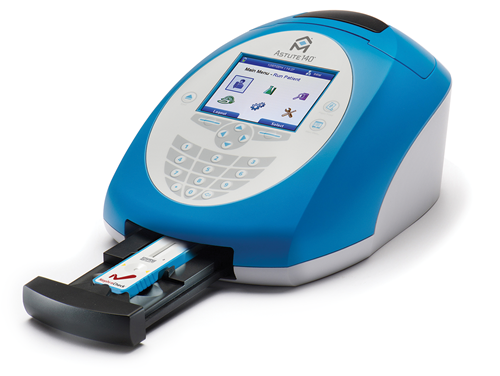
- Astute140® Meter Kit
- Astute140® Electronic Quality Control (EQC) device
- Nephrocheck® Test Kit
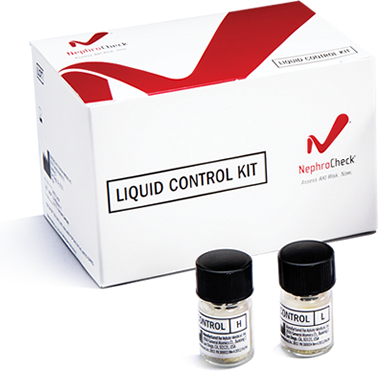
- NephroCheck® Liquid Control kit
- NephroCheck® Calibration Verification (CalVers) kit
(Available in the United States Only)